Quick-Start Synopsis
Across the pharmaceutical industry, there is a general uniformity in the organization of two of the primary business units (BUs): commercial and research and development (R&D). This is not the case for a third group, which may or may not be classified as a BU within an organization: Medical Affairs (MA). The structure of and functions within MA varies widely across the industry based on factors including:
- Company size
- Company maturity and stage of development of its products
- Portfolio and target consumers
- Senior leadership’s opinions of and previous experiences with MA
Because of these factors, the value an organization places on MA is also highly variable. Some perceive it to be a cost center populated mostly with high-priced FTEs (e.g. MSLs, Medical Directors) and activities (investigator-initiated studies, CME support) whose ROI is difficult to quantify. As the role and reach of MA has continued to expand over the past two decades, that viewpoint is slowly being replaced by a more favorable opinion about the positive impact MA can have on revenue and other core business objectives.
In this blog we will assume the role of a senior Medical Affairs executive who wants to definitively demonstrate the value MA brings to the organization. Let’s say this executive is putting together a presentation for the C-suite. The presentation will need to include the metrics the MA group is using to track performance and the executive must be prepared to explain how these metrics compare to other similar companies.
What are industry-standard metrics for pharmaceutical companies?
Field Medical Affairs
Usually the largest component of an MA team, field MA (FMA) serves critical functions externally and internally. One of the most important is that this group can be at the forefront of product launches. FMA can provide the “boots on the ground” to prepare the market and raise awareness of the disease. Unlike a sales team, FMA can support the early, pre-commercial phases of a product launch.
Traditional Metrics for FMA
Aka “hard” metrics, these can usually be quantified by number. Although these may look impressive on a department’s dashboard, they can encourage a culture of quantity over quality.

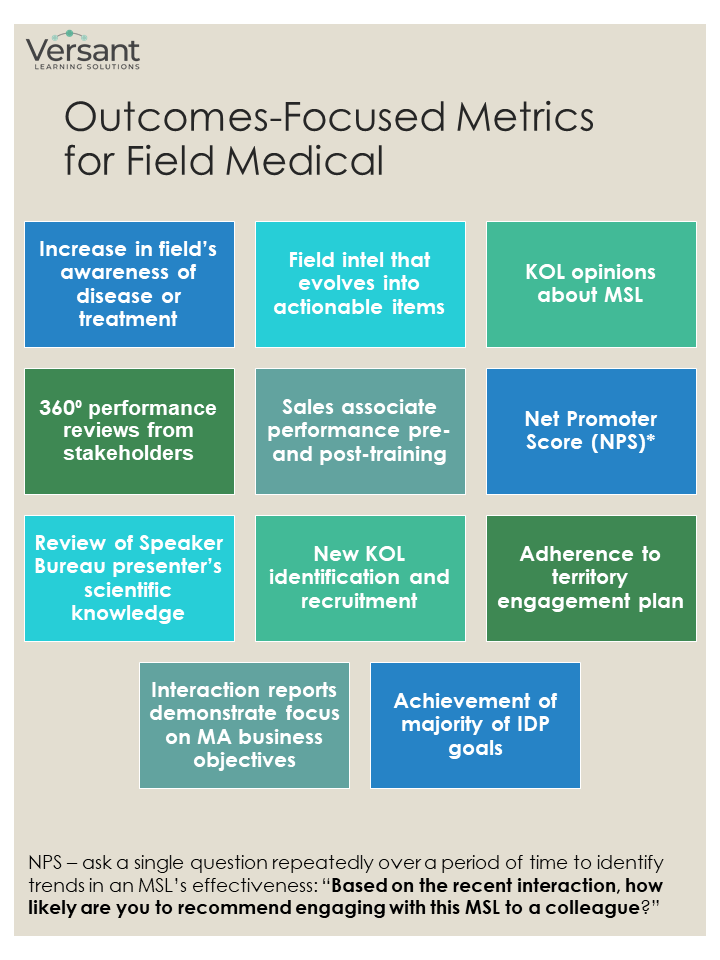
Outcomes-focused
Recognizing that traditional metrics may not accurately capture the impact of FMA on business objectives and revenue, some organizations are beginning to explore outcomes-focused metrics. As an example, one outcomes-focused metric is assessment of the growth of an MSL’s relationship with a KOL over a period of time. If that sounds difficult to do, you’re right.
Current Industry Norms for MSL Field Meetings
The table below is a summary of metric targets reported via industry surveys. These targets are highly variable and depend on the organization and its business objectives. Also, keep in mind, these data are pre-COVID 19.

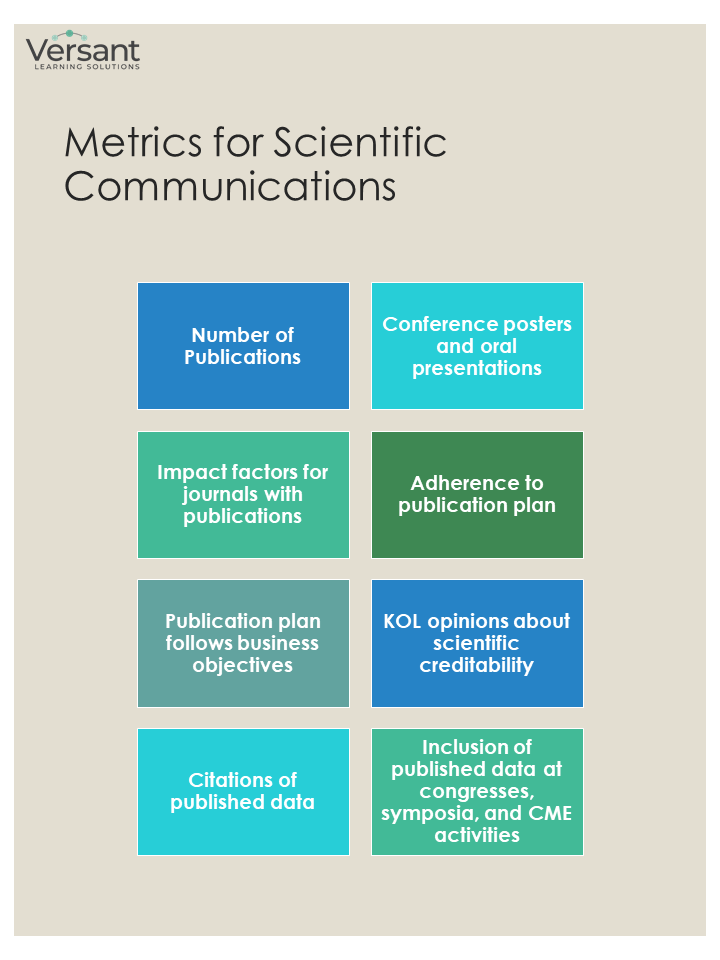
Scientific Communications
While FMA is largely responsible for communicating the data, it is Scientific Communications that generates most of the output. Medical Information (MI), which is responsible for creation and distribution of standard response letters (SRLs), may fall under the Scientific Communications umbrella or may function separately.
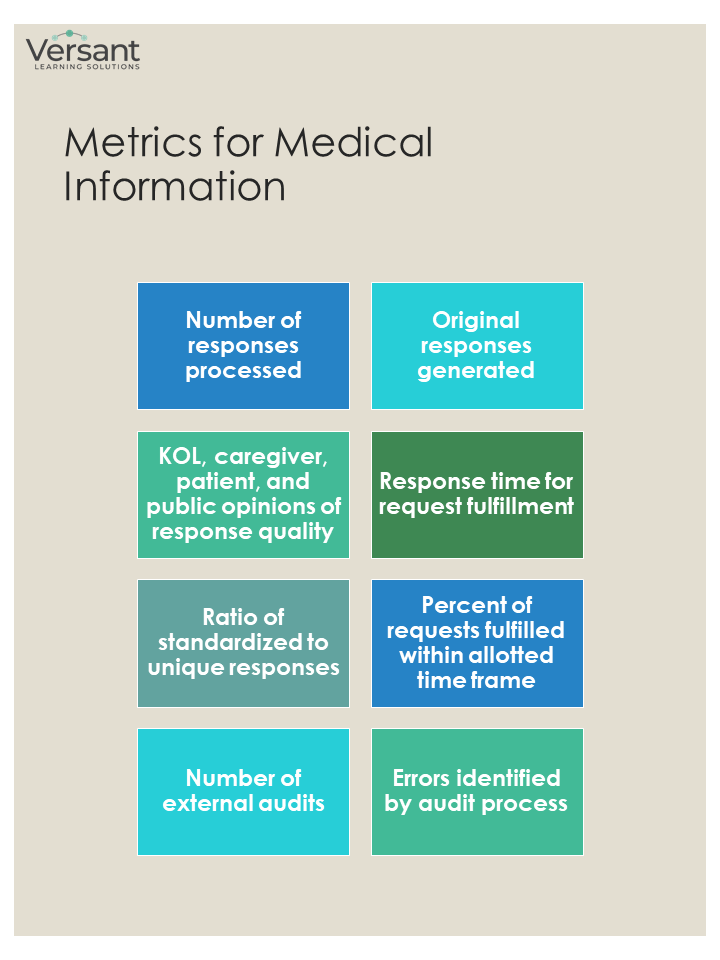
Medical Information
Unlike FMA, demonstrating the value of MI is not usually necessary for MA leadership due to its well-established role in providing customer-facing services. State and federal agencies mandate reporting of adverse events and other important information for which MI and Pharmacovigilance teams share responsibility. Innovations in MI include the increasing use of infographics and other data visualization methods to improve comprehension of SRLs (aka “walls of jargon-heavy text”). Improved digital technology includes chatbots and other sophisticated customer-support tools and improved websites with greater interactivity.
Other MA Functions
As we’ve already discussed, the organization of and functions within a MA department vary widely across the industry and, as such, we are not going to cover some functions that are less common to MA. These include 1) investigator-initiated studies, 2) CME, 3) health and economic outcomes research (HEOR), 4) operations, 5) project management, and 6) training.
Proven Value for Medical Affairs
So, let’s return to our MA executive who’s busy creating some nice-looking dashboards for the presentation about the value the MA group brings to the organization. While the data are important and displaying them clearly and concisely is equally important, there are some questions the executive should be thinking about and prepared to address during the presentation.
- Are these metrics capturing activities and initiatives that are directly tied to the business objectives?
- Do these metrics tell us whether a desired outcome/strategic objective has been achieved?
- Would cross-functional stakeholders agree that these metrics are important to capture?
- Is there accountability; when and by whom (e.g., who knows whether a team achieves its goals)?
If the presentation can address these questions in the affirmative, the it’s likely that this particular executive is leading a highly effective MA team.
Contact Us to Develop Your Strategy
References
- Piliero P, McCall T. Defining and Measuring the Value Drivers for Medical Affairs. 2020.
- McCall T, Piliero P, Shepard K, Kaur M, Smith T. Demonstrating and Measuring the Value of Medical Affairs Across the Lifecycle. MAPS 2019 Annual Meeting; 2019; New Orleans: MAPS.
- Elegant V, Piliero P, Bahadursingh K. How to measure the impact (contribution) of Medical Affairs to the organization? ; 2019.
- Internal Groups are Valuable Sources for Pharmaceutical Competitive Intelligence Function | FiercePharma. FiercePharma [Internet]. 2011:[52 p.]. Available from: https://www.fiercepharma.com/pharma/internal-groups-are-valuable-sources-for-pharmaceutical-competitive-intelligence-function.
- Matheis R, Hogben D, Greenway I. Metrics-driven innovation in medical communications. MAPS2019 Annual Meeting; 2019; New Orleans: MAPS.
- MSL Insights. The MSL KPI Disconnect: A Global Survey of MSL KPIs and Metrics. Medical Science Liaison Society; 2015, October 2018/11/24/.
- Dietlin T, Morris, K. Optimizing the Impact of the Medical Affairs Function. Campbell Alliance; 2009 11/23/2018.

